HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Science Stands Out in Our Most Engaging Cornerstone Stories of 2022

limjr [at] chop.edu (By Jillian Rose Lim)
No one could predict what 2022 would bring, but you can always count on another year of celebratory science at Children’s Hospital of Philadelphia Research Institute — science that challenges the status quo, optimizes outcomes for children, and breaks barriers for what’s deemed possible in the field of pediatrics.
This year marks our 100th year of such scientific inquiry. Since Joseph Stokes Jr. established CHOP’s very first lab in 1922, scientific journals and mainstream magazines alike have featured our breakthroughs, gathering an audience of patients, families, researchers, and other curious minds. We’re proud that in the last few years, we’ve cultivated another platform from which we can share our science with the world: social media.
This year, our social media followers engaged the most with Cornerstone stories that feature innovative breakthroughs such as novel immunotherapies and new imaging technologies, as well as artistic approaches to science and diversity-driven initiatives. As in previous years, we rounded up the top six stories that received the most engagements on our CHOP Research Facebook, Twitter, and LinkedIn channels. Posts about these stories were widely liked and shared, and with each story revealing the passion of its respective scientists, we’re not hard-pressed to find out why.
Gene Regulation Goes ‘Live’ With NIH Innovator Award

Lights, camera, action! A National Institutes of Health award given to Mustafa Mir, PhD, sets the stage for scientists to view in live action and with novel detail the early stage of embryonic development in fruit flies. Though Dr. Mir, who is assistant professor in the Center for Computational and Genomic Medicine at CHOP, received the prestigious NIH Director’s Innovator Award in 2021, our January 2022 Cornerstone story about his work reeled in some of the most Twitter engagements of the year.
With support from the award, part of the NIH Common Fund’s High Risk, High Reward Program, Dr. Mir and his lab will develop and apply new imaging technologies that can directly visualize and quantify how the regulation of gene expression is orchestrated inside live developing fruit fly embryos. Specifically, they will look at a period of growth called re-gastrulation embryonic development during which future body plans are laid out. The project will help scientists better understand how genes are switched on and off and could eventually inform novel therapies to prevent or repair defects that arise from aberrant gene expression in aging and diseases such as cancer, according to Dr. Mir.
Dr. Mir’s passion for his research project shines bright in this story and so, we’re not surprised that it sparked engagement with our readers, too.
“That’s what makes me tick — trying to unveil fundamental principles that govern all of life — not just human beings,” Dr. Mir said. “Transcription is fundamental to all life. It happens in every living thing: plants, bacteria, animals. If we can understand it better, then the potential technologies that may emerge is limitless. Our hope is that we can contribute to knowledge in a manner that can benefit humanity in both the short and long term.”

HHMI Fellowship Supports a Science Community of Inclusivity
A Cornerstone Q&A featuring a talented CHOP research trainees was another one of Twitter’s top engaged posts in 2022. Franklin Staback Rodriguez, a fourth-year PhD candidate in the Pathology lab of Janice Burkhardt, PhD, received the Howard Hughes Medical Institute (HHMI) Gilliam Fellowship for Advanced Study as part of a student-advisor pair with Dr. Burkhardt. In our Cornerstone conversation with Staback, he shared that the award has special significance as it contributes to the creation of an academic environment of opportunity, inclusivity, and acceptance.
“This award stands out because, in addition to supporting the student’s research and progress toward scientific leadership, it also supports mentor activities and institutional changes to create a healthy academic ecosystem,” Rodriguez said in the Q&A. “I see this fellowship as a fantastic opportunity to improve as a leader in STEM and as a scientist … It is a promise to be the best mentor and leader I can be in hope of paying forward all the advice and support others, like Dr. Burkhardt, have given me thus far.”
In the Burkhardt Lab, Rodriguez looks at immune cells through a cell-biological lens, exploring the molecular control of immune cell migration. Because correctly regulated migration is necessary for cells to develop and function during an immune response, better understanding the migration process can inform the development of better therapeutic tools. Rodriguez’s current project seeks to characterize the signaling pathways that allow T cells to navigate complex environments as they search the body for pathogens.
On top of some scintillating science, our Q&A with Rodriguez also touched on the new normal brought on by the COVID-19 pandemic.
“In 2020, despite what the world was going through, I had to finish my graduate coursework and find a thesis lab during a time when research was practically not happening, and then prepare for my PhD candidacy requirements,” Rodriguez said in the Q&A. “Thankfully, in my lab, in my department, and with Dr. Burkhardt, I found the energy, support, encouragement, and warmth that got me through some stressful and hectic months. That felt amazing, especially on a personal level and as the first of my family to do this.”
Read the full Q&A with Rodriguez on Cornerstone..
Outsmarting Immunotherapy Resistance in B-ALL
The wonders of immunotherapy have always been a popular topic for our readers, and this year was no different: Our Facebook followers loved Cornerstone’s coverage of research seeking to better understand why a patient’s body might resist immunotherapies like chimeric antigen receptor (CAR) T-cell therapy. The January 2022 story described a new study published by Andrei Thomas-Tikhonenko, PhD, chief of the Division of Cancer Pathology at CHOP, Sarah K. Tasian, MD, chief of the Hematologic Malignances Program at CHOP, and their colleagues in the journal Blood Cancer Discovery.
Drs. Thomas-Tikhonenko and Tasian had set out to explore why CAR T-cell therapies that target the protein CD22 failed in pediatric patients with B-lymphoblastic leukemia (B-ALL). In the study, they identified aberrant splicing of the messenger RNA (mRNA) encoding CD22 as a possible mechanism for this resistance.
In order for our genes to direct protein production, DNA, the rough blueprint of our genes, must first be transcribed into mRNA. During this transcription process, mRNA molecules are “edited” or spliced into continuous pieces: the gene’s “text” must be scanned, sensible pieces must be stitched together, and nonsensical pieces must be disposed. During aberrant splicing, however, that editing process becomes jumbled: Parts of the gene text can be skipped, while nonsensical sequences could be retained. The jumbled code could result in proteins with abnormal functions, new functions, or no function whatsoever. In this particular study, the researchers found aberrant splicing led to the downregulation of the CD22 antigen protein and the production of malignant cells resistant to the CD22-directed immunotherapy.
“Previous work demonstrated that aberrant splicing is involved in resistance to CD19-directed therapies, and our foundational finding here is that in B-ALL CD22 is mis-spliced just as profoundly, if not more profoundly, than CD19,” Dr. Tikhonenko said. “As CD22-directed immunotherapies emerge as potential frontline treatments for patients with B-ALL, we will have the opportunity to study the molecular mechanisms of CD22 aberrant splicing and its abnormal protein isoforms, which could possibly be used as predictive biomarkers — and possibly even treatment targets — for new CD22-directed immunotherapies.”
Read the full Cornerstone story.
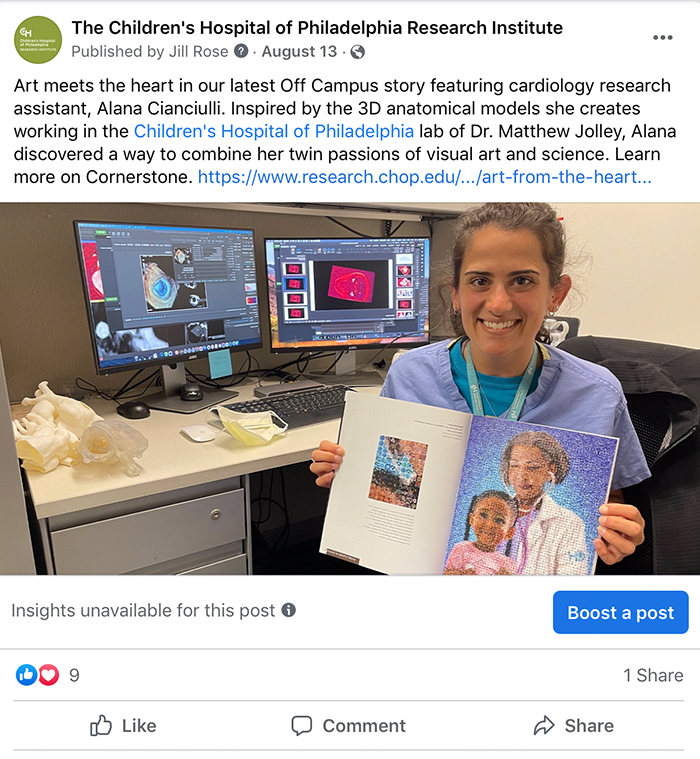
Art and the Heart Meet ‘Off Campus’
This year’s science stories weren’t confined to T cells and test tubes: In 2022, one of our Off Campus Cornerstone stories garnered the most engagements on Facebook for its entwining of scientific inquiry and amazing art. In “Art for the Heart,” we share the story of Alana Cianculli, former research assistant (and now consultant) in the lab of Matthew Jolley, MD, assistant professor in the Division of Cardiology. Working in the Jolley Lab, Cianculli was inspired by the 3D images of the heart she created using advanced computer programs.
“I had two loves — art, then science and medicine — and working with Dr. Jolley was where I was able to blend these passions together,” said Cianciulli, who is now also a medical student at Philadelphia College of Osteopathic Medicine. “In the lab, we create visual representations of heart models working with patient-specific anatomy, which fuels my creative ability.”
In the Jolley Lab, researchers visualize and quantify abnormal heart structures using multi-modality 3D imaging. Tech tools like SlicerHeart (co-developed by Dr. Jolley) allow them to build virtual or 3D-printed anatomical models of patients’ hearts. Since children with congenital heart disease can have a broad and unique range of anatomy, the customizable nature of the program is critical. Few tools are readily available for clinicians to conduct image-based structural phenotyping or patient-specific planning of interventions.
Cianciulli, who possesses an acute eye for the visual and a special spark of creativity, was moved by the models she creates and, along with a colleague, they conceived of a mosaic made up of miniscule images of virtual and physical models created by the lab. The resulting piece was published in Diastole, CHOP’s literary and arts magazine and will soon hang in physical form in CHOP’s Cardiac Center, where it’s sure to inspire both scientists and artists alike.
Read the full Off Campus story.

CAR T-Cell Therapies: Looking Back, Looking Ahead
Another CAR T-cell story proved popular for our LinkedIn readers this year. In “Where Discovery Leads: Taking CAR T-Cell Therapy to Solid Tumors and Beyond” on Cornerstone, we celebrated 10 years since the first pediatric patient, Emily Whitehead, received CAR T-cell therapy for relapsed acute lymphoblastic leukemia (ALL). Emily’s engineered T-cells have endured for a decade, and today she remains cancer-free. Since then, thousands of pediatric patients have received CAR T-cell therapy for relapsed ALL around the world.
On Cornerstone, we celebrated this important milestone by looking ahead at the future of CAR T-cell therapies. At CHOP, researchers continue to work diligently to harness the immunotherapy’s power for other childhood cancers including brain tumors, neuroblastoma, and acute myelogenous leukemia, as well as to discover ways to improve how CAR T delivery works in the body.
In the story, we included short videos from Dr. Tasian and Jessica Foster, MD, attending physician in the Division of Oncology. You’ll see Dr. Tasian discuss the development of novel immunotherapies for acute myeloid leukemia, an aggressive blood cancer with a high risk of relapse and poor prognosis in children. Meanwhile, Dr. Foster explores alternative ways to deliver CAR T cells directly to brain tumors while sparing healthy brain tissue.
The popular story also covers innovative research from John Maris, MD, Giulio D’Angio Chair in Neuroblastoma Research and Shannon Maude, MD, PhD, attending physician in the Cancer Center. As co-leader of the Pediatric Cancer Dream Team, Dr. Maris seeks to identify immunotherapeutic targets for hard-to-treat childhood cancers like neuroblastoma. One such target, GPC2, an antigenic protein expressed on the surfaces of neuroblastoma, will be explored in clinical trials, the first of which will open this year.
Meanwhile, Dr. Maude works on determining why some patients’ CAR T cells last for years, while others’ CAR T cells last for less than a few months. One such reason could be the human body’s recognition of CAR T cells as foreign; Dr. Maude and her team thus altered the antibody to make it appear more like a human protein by changing some of the DNA sequences.
Read the Where Discovery Leads story on Cornerstone.

Making Space for Science: Welcome Morgan Center!
The final story for our most engaging stories is, quite fittingly, a story about the future. In October 2022, developers broke ground on the newest home for CHOP’s research breakthroughs, the Morgan Center for Research and Innovation. We covered the groundbreaking ceremony celebrating both the Research Institute’s expansion and our 100th anniversary in a Cornerstone story that drew in some of the most LinkedIn engagements of the year.
The ceremony was attended by Doug Hock, CHOP executive vice president and chief operating officer; Donald Moore, senior vice president of real estate, facilities, and operations; Joseph St. Geme, MD, physician-in-chief; Susan Furth, MD, PhD, chief scientific officer; Beverly Davidson, PhD, chief scientific strategy officer; as well as Pennsylvania State Representative Jordan Harris.
“I am honored and grateful to be here supporting this project,” Harris said during the ceremony. “Thank you for making this healthcare system the world-class system that it is: a world-class system that will help our most valuable resource in our country — and that is our children.”
The Morgan Center will stand at 14 stories with 350,000-square feet of space devoted to wet and dry labs, as well as office and retail space. Described as “an important part of our strategy to advance research at CHOP” by Hock, the building was designed with a layout to encourage collaboration and flexibility; wet labs will be situated next to dry labs to stimulate a more seamless exchange of ideas and communication.