HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Emily Whitehead, 10 Years Later: Q&A With Stephan Grupp, MD, PhD

Emily Whitehead, the first pediatric patient to receive CAR T cell therapy, is celebrating 10 years cancer-free.
Ten years ago, Emily Whitehead came to Children’s Hospital of Philadelphia with her parents, Tom and Kari, “looking for a miracle” after relapsing with acute lymphoblastic leukemia (ALL). Little did she and her family know that Emily would become an international phenomenon, as the first pediatric patient to receive CAR T-cell therapy.
Her medical team rallied to help her overcome an initial severe reaction, and then she went into remission within days after receiving the therapy. Today, Emily is 17 years old planning for a bright future. Watch a video about Emily here.
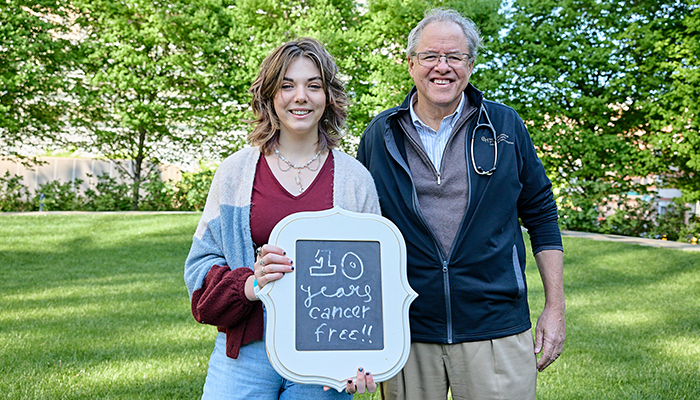
Dr. Stephan Grupp, here with Emily, administered the therapy to Emily 10 years ago.
Stephan Grupp, MD, PhD, section chief of the Cellular Therapy and Transplant Section at CHOP, as well as director of the Susan S. and Stephen P. Kelly Center for Cancer Immunotherapy and the Yetta Deitch Novotney Endowed Chair in Pediatric Oncology, delivered those CAR T cells to Emily. He played a critical role in pediatric research of CAR T-cell therapy, paving the way for its approval by the U.S. Food and Drug Administration in 2017. Now called tisagenlecleucel or Kymriah, it was the first CAR-T treatment in the world, as well as the first gene therapy in the U.S. CHOP has since treated more than 440 patients with this therapy, and thousands of pediatric patients around the world have received it as well
In a Q&A, Dr. Grupp reflects on his experience with Emily and what researchers learned from her.
Q: When you gave Emily the CAR-T cell infusion 10 years ago, what were your hopes for therapy?
A: Well, hope and realistic expectation are sometimes two completely different things. The realistic expectation would have been that it provided some degree of disease control. In the back of my mind was that this was a Phase 1 trial for a new oncology agent. Across all such trials (kids and adults), there was about a 1% to 3% chance that there would be significant benefit. Our hope was that we could get her into remission long enough to have a transplant. But then we shot right past even our most outrageous hopes to something completely different —which was that those cells were sticking around. Not only did they put her in remission, but they stayed in her body. Then the hope was that they could stay and prevent relapse for a longer period.
Q: What is it like, as a scientist and a doctor who’s been through this journey with her, to see Emily as a 17-year-old?
A: Seeing my patients grow up is simply the best thing ever. From Emily’s standpoint, she is the international face of CAR-T cell therapy. She and her family are involved in incredible advocacy work, and they are a clearinghouse for information. Families struggling with cancer reach out to them all the time. Emily is a great young lady, talking about college and future plans. It has been an amazing journey with her and her family.
Q:What did Emily Whitehead’s experience teach you and your team about CAR-T cell therapy?
A: Everything. Emily was the first pediatric patient who we ever treated with CAR T cells. She had no therapeutic options and had been told she should go to hospice. We got the trial open the second she needed it. And what we learned from Emily really has defined the entire field of CAR-T cell therapy, without a doubt.
She developed cytokine release syndrome, which we now know to be the most significant and common toxicity of CAR-T cell therapy. That complication was nearly fatal to her. At that point, we had run out of things to do for her. On the fly, and with the amazing cooperation of the team at the University of Pennsylvania, we made the discovery that a particular inflammatory molecule, interleukin-6, was very, very high in her. Fortunately, at that time in 2012, there was a drug, used for arthritis patients, that could block interleukin-6. No one had ever used it for this purpose. No one had ever used it in a cancer patient that we were aware of. This is just something that we thought might help her based on the data, and it did in an unbelievably dramatic way. What we learned really was how to control this toxicity when it gets serious. That got disseminated very quickly across the field and was incorporated into all the ongoing trials. It’s now the only approved treatment for this complication.
Overnight, she went from somebody who was critically ill, on the point of probably succumbing, to basically becoming stable and not requiring medicine for blood pressure support anymore, decreasing the amount of respiratory support she needed, decreasing the amount of assistance needed, decreasing those pumps that she needed. This was within hours. She recovered very quickly after that and was in remission when we checked her bone marrow, 23 days after we gave her the CAR T cells.

Emily and her parents, Tom and Kari, are now international advocates for research funding for innovative pediatric cancer treatments.
Q:What is it like to be the first to treat not just any patient, but a pediatric patient, and having these conversations with her parents about completely uncharted territory?
A: We had mentioned to the Whiteheads the possibility of Emily getting sick in ways that we didn’t expect, but we certainly didn’t warn about cytokine release syndrome because that wasn’t even a phrase that we knew at the time. We wanted to help Emily, and we felt there was an excellent chance to learn things that would help other kids, but we had no idea what to expect. For me, the feeling that I remember having at the time was, having given those cells to her with my own hands, I felt this enormous sense of responsibility. She had gotten so sick, and it was something that I had done.
Q: There’s a huge team of people who have been involved in developing CAR-T cell therapy. Can you talk about some of the different members of that team?
A: Well, the most important thing to say is that this was a collaboration with Carl June’s group at the University of Pennsylvania who developed the actual CAR T molecule. David Porter, who runs the Cell Therapy Transplant Group over at Penn Medicine’s Abramson Cancer Center — in the same way that I run the Cellular Therapy and Transplant Section at CHOP — and I have been collaborating for many years. We trained together in Boston. He treated the first adult patients with chronic lymphocytic leukemia at the University of Pennsylvania.
Sue Rheingold, in the leukemia group in CHOP’s Division of Oncology, was very involved with the Whitehead family when they first came to CHOP. Shannon Maude has been an invaluable cell therapy colleague running important trials since the early days of our program. More recently, Amanda DiNofia, a physician in the Cancer Center, was critical in developing CHOP INDs to open protocols at CHOP. Then there was a huge collaboration with Novartis for the commercial roll-out, where I had the opportunity and privilege to run the Steering Committee for the international registration trial.
The Clinical Vector Core at CHOP had an enormous contribution, developing the clinical vector that is used to genetically modify the cells. This is the key ingredient in making these cells. Multiple studies used the CHOP vector for their CAR T-cell studies, and we continue to use the CHOP vector in our research studies. It is a unique resource, and it is right here at CHOP.
Q: What can we say about survival rates for ALL patients who receive CAR-T cell therapy, knowing that it’s a different experience for every patient?
A: There’s a lot of variability here. With ALL, we published a study on earlier treatment of cytokine release syndrome that included 70-plus patients; the likelihood of those patients going into remission was 97%. Even looking at the group of patients who had completely uncontrolled disease like Emily did, the likelihood of those patients going into remission was 87%. That’s our ability to get patients to remission — but that’s not what the families are looking for. They want long-term disease control, ideally without having to go to transplant. We’re updating all these data right now, but what we have published so far would indicate that there is about a 50% likelihood of staying in long-term remission. And when I say long term, I mean years. So obviously we need to follow up more and more patients for a longer and longer time.
Q: You’ve been cautious about calling this a “cure.” Are you able to call it a cure now?
A: We actually refer to it as the ‘C word.’ But now we're 10 years out. Do I believe Emily is cured? Do I believe other children who are five or nine years out from their CAR-T therapy are cured? I believe they are.
Read more in the CHOP press release.


