HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Building Better Strategies to Protect the Brain From HIV
 By Barbara Drosey, Jillian Rose Lim, Nancy McCann, and Sharlene George
By Barbara Drosey, Jillian Rose Lim, Nancy McCann, and Sharlene George
Editor's Note: Where Discovery Leads is a multimedia storytelling project that delves into key research themes at CHOP Research Institute. This is part one of a two-part series that focuses on the suite of scientific studies aimed at better understanding the neuropathogenesis of HIV infection on cognitive and intellectual development. See part two of this series.
Fear and panic have transformed into hope and promise over almost four decades of human immunodeficiency virus (HIV) research. New combinations of antiretroviral therapy (ART) and early testing methodologies offer opportunities for a durable, functional cure for people living with HIV. They can pursue active, fulfilling lives and have children and partners who are HIV-negative, a feat that seemed unimaginable when the first cases of HIV/AIDS were reported in the early 1980s and spiraled into an epidemic.
Yet a sense of urgency remains for scientists committed to unravelling the mysterious and menacing retrovirus that stays hidden in a reservoir in HIV-infected individuals' bodies, but exactly in which cells it takes up residence is unknown.
"The virus is, sadly, probably smarter than we are," said Steven D. Douglas, MD, professor of pediatrics and director of Clinical Immunology Laboratories at Children's Hospital of Philadelphia. (View a short video interview with Dr. Douglas.)
Dr. Douglas and his colleagues at CHOP remain undaunted. They are probing the complexities of HIV from multiple vantage points at cellular, mechanistic, and behavioral levels, concentrating on how HIV has insidious effects on the brain. HIV associated neurocognitive disorder (HAND) persists in nearly 50 percent of both children and adults infected with the virus, causing cognitive, behavioral, and motor deficits, even when ART keeps the virus under control.
"Now that people are surviving HIV very routinely, the question shifts to how do we maximize people's health potential?" said Stewart Anderson, MD, director of research for the Department of Child and Adolescent Psychiatry and Behavioral Sciences at CHOP and co-director of the Lifespan Brain Institute, a collaboration between CHOP and the University of Pennsylvania. "One of the areas that we clearly need to do better in is preserving infected children's cognitive and intellectual development."
The Sooner the Better for ART
With more opportunities now available for early HIV testing, clinicians' first visit with patients living with HIV is usually much closer to their time of infection than in the past. Scientists don't know exactly at what point neurocognitive effects begin, but they believe the most protective intervention is to start ART as soon as possible.
Sarah Wood, MD, MSHP, an attending physician who provides medical care to youth living with HIV at the CHOP Adolescent Initiative clinic, uses a detailed psychosocial history to identify existing depression and trauma to help her team understand how to best support each patient. Whether that involves linking someone to therapy, substance abuse treatment, or prescribing an antidepressant, it always means starting patients on an HIV treatment regimen immediately upon diagnosis and encouraging their medication adherence to mitigate the risk of neuro-inflammation contributing to mental health problems and depression.
No matter their situation, Dr. Wood's patients receive a message of hope.
“I want them to leave here knowing they are going to have a long and healthy life,” said Dr. Wood, whose research focuses primarily on improving access and adherence to HIV pre-exposure prophylaxis. “All the things they thought they were going to do before HIV are still the things they are going to do after HIV, contingent on taking medication every day.”
Study: Young People With HIV Access More Mental Health Services
Evaluating neurocognitive outcomes using data from the Pediatric HIV/AIDS Cohort Study (PHACS) group, Richard Rutstein, MD, pediatrician and medical director of the Special Immunology Service at CHOP, and colleagues published the results of a study examining the prevalence of mental health diagnoses, clinically significant symptoms, and utilization of mental health treatment services among perinatally HIV infected (PHIV) and perinatally HIV exposed but uninfected (PHEU) young people ages 10 to 22.
Although perinatal HIV infection in the U.S. is becoming a rare occurrence, Dr. Rutstein said, "There remains a cohort of perinatally HIV-infected children and perinatally exposed but uninfected children, and as they age through adolescence and into adulthood, we need to remain cognizant of the fact that they have a slightly higher risk of psychosocial and educational issues."
Along with his co-authors, Dr. Rutstein found a convergence of factors contribute to mental health diagnoses such as stressful life events (to which youth living with HIV have a greater chance of being exposed); caregiver and family relationships, health status, and resources; socioeconomic status; and ethnicity. The fact that more young patients with HIV access mental health services than their uninfected peers (67 percent versus 51 percent) might simply be a residual effect of their frequent interaction with healthcare services, which are often funded through government programs.
Yet, Dr. Rutstein, who is interested in whether HIV-infected individuals' neurocognitive outcomes change based on early diagnosis and early access to ART, suspects a deeper biological undercurrent also plays a role. In previous studies, researchers realized that children infected with HIV had subtle neurocognitive defects — even without progressive encephalopathy — and their IQs and learning abilities were not what you would find in a normalized general population.
White Matter, Myelin, and Neurocognitive Decline
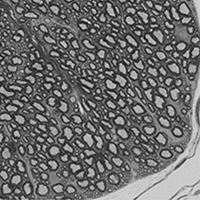
Myelin wrapped around nerve processes in mouse spinal cord. Image courtesy Judith Grinspan, PhD
One of the most consistent things seen in the brains of people with HAND is a loss of white matter, said Judith Grinspan, PhD, research scientist at CHOP and professor of Neurology at the Perelman School of Medicine at Penn. White matter gets it color from myelin, the fatty membrane (70 percent lipid) that coats the nerves and is necessary for the conduction of nervous impulses as well as protecting the nerve. HIV-infected children can have abnormal white matter and irregularities of the myelin sheath, Dr. Grinspan explained. Myelination is rapid during infancy but continues through adolescence into early adulthood.
"Generation of myelin sheath is especially critical in the pediatric population in which myelination is in progress and is necessary to establish critical connections that regulate motor control, cognition, and behavior," Dr. Grinspan said.
As co-principal investigators of a NIH grant, Dr. Grinspan and Kelly Jordan-Sciutto, PhD, director of Biomedical Graduate Studies at the Perelman School of Medicine and professor of Pathology at the School of Dental Medicine, both at Penn, hypothesize that abnormalities in white matter and myelin contribute to the neurocognitive decline that's associated with HIV. Their study uses tissue culture models and disease models to assess the effects of both the HIV infection and ART on cells in the brain called oligodendrocytes, which means “cells with a few branches,” that are essential to the maturation and development of myelin.
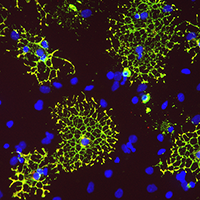
Oligodendrocytes growing in a petri dish. Image courtesy Judith Grinspan, PhD
To date, they have tested several ART drugs, with more to go. Their goal is to alert clinicians to which drugs may affect myelin so they then can take this into consideration when prescribing these drugs to patients, particularly children and adolescents who are rapidly producing myelin due to brain development.
"I'm always a little worried that someone is going to think that we're saying the antiretrovirals are bad," Dr. Grinspan said. "Not at all! We’re trying to refine them in order to find treatments that are less harsh."
As far as how the virus, itself, affects oligodendrocytes — it's an indirect process. The virus infects immune cells, which essentially makes them angry. They secrete harmful products that can either destroy the oligodendrocytes or stop them from maturing. Once the myelin starts to degenerate and leaves the nerve without a covering, the nerve will start to fall apart. If the neuron is destroyed, the myelin will also fall apart. They are dependent on each other, so the challenge for the research team is to decipher if the neuron damage leads to secondary white matter loss, or if white matter loss leads to secondary neuron dysfunction.
Dynamic Testing Ground
In another collaborative effort, Dr. Jordan-Sciutto's lab, Dr. Anderson's lab, and the Human Pluripotent Stem Cell Core at CHOP directed by Deborah French, PhD, have developed the first entirely human system where scientists can study three cell types in the central nervous system (CNS) within the context of HIV infection.
"We now have a system in a dish where we can go after new kinds of approaches to limit the virus' negative effects," Dr. Anderson said.
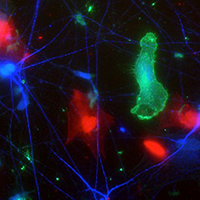
An HIV+ human stem cell derived microglia (green) grows in culture with human stem cell derived neurons (blue) and astrocytes (red). Image courtesy Sean Ryan, University of Pennsylvania Perelman School of Medicine and Graduate Student in the Cellular and Molecular Biology program.
This brain-like environment inside a petri dish is populated by neurons, astrocytes, and microglia grown from human-induced pluripotent stem cells (HiPC). The tri-culture system is giving researchers powerful insights into a frontline question: Why do HIV-infected patients who take antiretroviral drugs experience a cognitive decline and psychiatric diagnoses?
"There is a concern that being on the drugs long-term may be causing some of the dysfunction, or perhaps it's the virus, or some combination," Dr. Jordan-Sciutto said. "This model really allows us to ask that question in a reduced form. We can look directly at HIV infection, at the drugs themselves, and the drugs in the context of infection."
Because the stem cells are taken from patients, Drs. Jordan-Sciutto and Anderson can also look specifically at how HIV infection in the CNS behaves across individuals with different genetic backgrounds. And studying in vitro culture allows the researchers to test things mechanistically with many manipulations; unlike studies in people, these tests can say that something is indeed a cause of the dysfunction rather than an association.
The researchers have used this dynamic testing ground to glean some important insights into why post-mortem studies of deceased individuals with HIV show a decrease in synapses between brain cells. The team observed that infected microglia, the major inflammatory cells in the brain, release cytokines, particularly one named interleukin 6 (IL-6) to alert other immune cells of an infection. Now that the team can see this inflammation is going on, they can use the dish model to explore further and test treatments.
“What we wanted to test is what the HIV-infected microglia are releasing, and perhaps those compounds will affect the other cells in the dish, making them more likely to eat synapses,” Dr. Anderson said. “On top of that, if causal effects of the infection on neuronal connectivity or health are established, our system is ideal for the high-throughput testing of medication, antioxidant, or other approaches to preventing or correcting these effects.”
Biomarkers of HIV's Effects on the Developing Brain
Very little is known about the effects of acquiring HIV during adolescence on normal brain maturation, which typically includes the development of problem-solving, complex reasoning, and self-control. By using computational neuroimaging metrics — MRI data that can be quantified, like brain volume or signal intensity — Jennifer McGuire, MD, MSCE aims to identify reliable biomarkers that will allow her to develop mechanistic hypotheses about how HIV is interfering with brain development and how neurologic effects of HIV may differ in adolescents compared to adults.
Dr. McGuire, a neurologist and epidemiologist at CHOP and an assistant professor of Neurology and Pediatrics in the Perelman School of Medicine at Penn, is conducting her research in a cross-sectional study of 16 to 20-year-old male and female Philadelphia youth with behaviorally acquired HIV compared to uninfected controls matched by age, sex, demographic, and HIV risk factors. Her study is the first to examine the structural and functional brain differences of this age group in HIV.
The first portion of the MRI measures the volume of different structures in the brain and looks at white matter integrity — the connections between the cells and how their brains are networked. The second portion is fMRI, which involves presenting participants with a cognitive test or game to play while they're in the scanner, measuring regional blood flow to identify what portions of the brain are activated while doing the task. This reveals both the accuracy with which they do the task, as well as how hard they are working to do it on a cellular level.
Once they've completed the initial study, Dr. McGuire plans to do a follow-up for all who are interested. Having two time points to compare how brain volumes or functions may change within an individual will help her understand if differences from uninfected controls are due to a failure of age-expected maturation, active brain damage, or both.
"Understanding the neurologic effects of HIV in adolescents, and how they may differ from what has been described in adults, is critical to developing long-term treatment strategies to help this very vulnerable population," Dr. McGuire said. "The ultimate purpose of this study is to determine if there is a way we can specifically intervene to improve the quality of life of adolescents living with HIV for years to come."
NeuroAIDS Project: Looking for Novel Therapeutics
The holy grail of HIV research, according to Dr. Douglas, is to pinpoint the reservoir where HIV hides and ensure it doesn’t resurrect. With support from the NIH-funded NeuroAIDS grant, Dr. Douglas and co-principal investigator Jay Rappaport, PhD, professor of neuroscience and neurovirology at Temple University School of Medicine, sought new methods to eradicate HIV in brain cells that evade conventional antiviral treatments.
"The non-replicating, latent virus wakes up, and we will see a re-emergence of virus in a patient who had gone quiescent for years, then has a flu or has some other infection," Dr. Douglas said. "Some think stress or mood disorders may play a role, but the short answer is we don't know."
The study team targeted different biological pathways crucial to the persistence of HIV/AIDS. Dr. Douglas already had demonstrated that the FDA approved drug aprepitant, a high-affinity antagonist of substance P (a neuropeptide with a key role in promoting inflammation during HIV infection), demonstrates antiviral activity by blocking inflammation and neurokinin-1R, a cell receptor that binds to substance P. They hypothesize that manipulating neurokinin-1R can disrupt HIV’s entry into cell reservoirs and block the viral replication that accounts for HIV’s devastating effects. By better understanding the neuropathogenesis of HIV/AIDS, they seek to develop novel therapies that could one day eliminate the formation HIV reservoirs.
The NeuroAIDS investigators also studied the role of substance P in HIV infection in the CNS. The unique environment in the CNS, when combined with HIV infection, promotes inflammatory conditions favorable for HIV replication and survival of HIV-infected macrophages, creating a continuous HIV reservoir. By targeting pathways to disrupt HIV infection in CNS macrophages, they aim to find new approaches to interfere with HIV replication.
As the NeuroAIDS project comes to a close, Dr. Douglas and colleagues are already pursuing a new grant to continue their work. "After 30 years on this path, I still have questions I want to answer," he said.


