HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
In This Section
Researchers Explore Connection Between Mitochondrial Function and Schizophrenia in 22q11DS
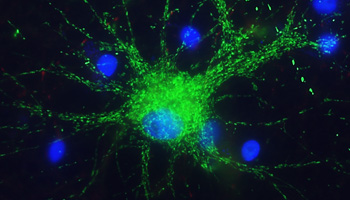
Astrocytes, the most numerous cell type in the central nervous system, perform various tasks, including synaptic support such as regulating neuronal signaling.
A multidisciplinary team of researchers from Children's Hospital of Philadelphia Research Institute and the University of Pennsylvania identified a potential approach for targeted preventive therapy and treatment for schizophrenia in patients with 22q11.2 deletion syndrome (22q11DS).
Individuals with 22q11Ds are approximately 25 times more likely to develop schizophrenia than the general population, whose lifetime risk is about 1 percent. They also are susceptible to myriad health conditions, developmental delays, and behavioral health challenges.
"From my perspective, studying schizophrenia and 22q is helpful because if a quarter of the children with this condition are going to develop schizophrenia, we have a much better chance of trying to figure out how it is happening and if there is a way to prevent it," said Stewart Anderson, MD, director of Research in the Department of Child and Adolescent Psychiatry and Behavioral Services at CHOP and associate director of the Lifespan Brain Institute at CHOP and Penn.
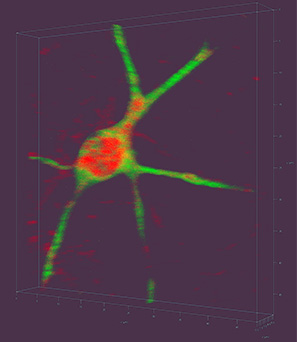
Researchers measured mitochondrial function in induced pluripotent stem cell-derived neurons from control individuals and from adults with 22q11DS.
The study team's paper published in JAMA Psychiatry compared measures of mitochondrial function and the expression of related genes in 14 induced pluripotent stem cell-derived (IPSC) neurons from typically developing control individuals and from adults with 22q11DS. The individuals with 22q11DS comprised two groups — those diagnosed with schizophrenia and those without this diagnosis. Similarly, the study team measured lymphoblastic cells lines (LCLs) from a separate group of adults with 22q11DS with or without schizophrenia.
The IPSC lines were derived from individuals with 22q11DS at Stanford University and the LCLs were from adults within the 22q and You Center at CHOP. Elaine Zackai, MD, director of Clinical Genetics at CHOP and professor of Pediatrics in Genetics at Penn, Donna McDonald-McGinn, MS, CGC, director of the 22q and You Center and associate director of the Clinical Genetics Center at CHOP, and the lab of Beverly Emanuel, PhD, CHOP investigator, professor of Genetics, and Charles E.H. Upham Endowed Chair in Pediatric Medicine at Penn, have been diligently storing LCLs from 22q clinic patients from pediatrics through adulthood for the past 20 years. Raquel Gur, MD, PhD, the Karl and Linda Rickels Professor of Psychiatry and director of the Lifespan Brain Institute at CHOP and Penn, and her team have been following many of these patients with research-grade neurocognitive assessments and other studies, including brain imaging in many instances, for years.
In an earlier proof-of-principle study, Dr. Anderson's team studied stem cell-derived neurons and demonstrated that, compared with healthy controls, patients with 22q and schizophrenia had mitochondrial dysfunction; however, that study did not account for 22q patients who had not developed schizophrenia.
"In the current paper, we show not just with the IPSCs but also with these LCLs that you can reliably distinguish the presence or absence of schizophrenia in individuals with 22q11DS by evaluating the mitochondria function of their blood cells," Dr. Anderson said. "We hope that with further study these findings will grow into actionable science whereby we can predict, and even prevent, the development of schizophrenia in adolescents with 22q11.2 deletion syndrome."
When looking at mitochondria genes, the researchers focused on levels of adenosine triphosphate (ATP), a major energy source for cells produced by mitochondria. ATP levels were not reduced in patients with 22q11DS who were not diagnosed with schizophrenia; however, the expression of multiple genes encoding for oxidative phosphorylation, a process that helps produce ATP, was upregulated compared with both the 22q11DS with schizophrenia group and the control group.
These findings suggest that increased mitochondrial biogenesis is associated with the absence of schizophrenia in 22q11DS. Therefore, the investigators hypothesize that the enhancement of mitochondrial biogenesis may provide a targetable opportunity for treatment or prevention of this disorder in individuals with 22q11DS.
Indeed, in the IPSC-derived neurons from individuals with 22q11DS and schizophrenia, their mitochondrial ATP production was normalized by exposure to an FDA-approved medication.
"We just started a National Institutes of Mental Health-funded study that will allow us to determine whether weaker mitochondrial ATP generation in LCLs from teenagers with 22q, who did not have schizophrenia at the time their blood sample was taken, and who have been followed by Dr. Gur's team and the 22q and You clinic into young adulthood, predicts their likelihood of developing schizophrenia," Dr. Anderson said. "If so, we will have compelling rationale for a preventive trial in the highest risk 22q adolescents using one of the FDA-approved enhancers of mitochondrial biogenesis."
A Purposeful Match
It's no accident that Dr. Anderson found his research home 10 years ago at Children's Hospital of Philadelphia, which has the world's largest clinic following and studying patients with 22q11DS. While 22q11DS occurs in about one in 3,000 births, it is considered a rare disease, affecting approximately 100,000 people in the United States.
"We focus on this condition in our research because it is a defined genetic disorder in which six of the 46 deleted genes encode for mitochondrial localizing proteins," Dr. Anderson said. "Based on the literature, there's good reason to believe, in autism and in schizophrenia generally, that mitochondrial abnormalities can contribute to the illness."
Dr. Anderson credits colleague Douglas Wallace, PhD, director of the Center for Mitochondrial and Epigenomic Medicine, with suggesting that enhanced mitochondrial function may protect most 22q11DS patients from developing schizophrenia.
"When we showed him this gene expression abnormality where the no-schizophrenia group showed an upregulation of mitochondria genes, he pointed me toward a mitochondria illness called Leber's hereditary optic neuropathy," Dr. Anderson said.
About half the people with the gene that causes Leber's experience loss of central vision and, ultimately, blindness. Those with the condition who do not lose vision show a similar pattern of enhanced mitochondrial gene expression.
"Interestingly, the blindness develops in adolescence, similar to the initial symptoms of schizophrenia," Dr. Anderson added.
In addition to McDonald-McGuin, Dr. Zackai, and Dr. Emanuel, first author Jianping Li, PhD, postdoctoral fellow; Oanh Tran, research technician; T. Blaine Crowley, research data analyst; and Tyler Moore, PhD, psychometrician, also are co-authors on the paper.
This study built upon previous work in collaboration with Douglas Coulter, PhD, CHOP investigator and professor of Pediatrics at Penn, in which focal cell type specific gene therapy was applied in an animal model to correct behavior deficits that are related to autism and schizophrenia.
Read the CHOP press release for additional information.