HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
St. Geme Laboratory Research Overview
The St. Geme Lab focuses on H. influenzae and K. kingae as model pathobionts and is using genetic methods, protein chemistry, carbohydrate chemistry, X-ray crystallography, high-resolution microscopy, cell biology approaches, and animal models to study the molecular and cellular determinants of H. influenzae and K. kingae disease. Learn more about the research of Dr. St. Geme and his team:
Kingella kingae
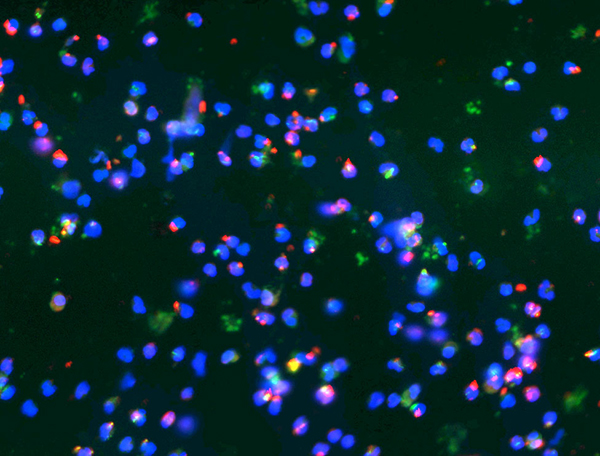
Kingella kingae is an emerging pediatric pathogen commonly found in the oropharynx of children ages 6 to 48 months. A subset of colonized children develop severe invasive disease, characterized by osteoarticular infection, bacteremia, and endocarditis.
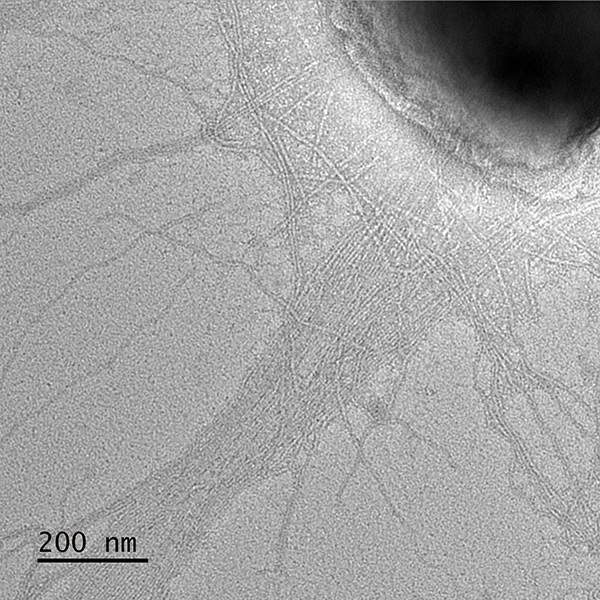
The development of invasive disease is facilitated by several pathogenicity determinants, including a polysaccharide capsule, an exopolysaccharide, type IV pili, and an RTX-family cytotoxin. Together, these factors allow the bacterium to adhere to and lyse host cells, survive challenge by the immune system, and disseminate to distant sites to produce disease.
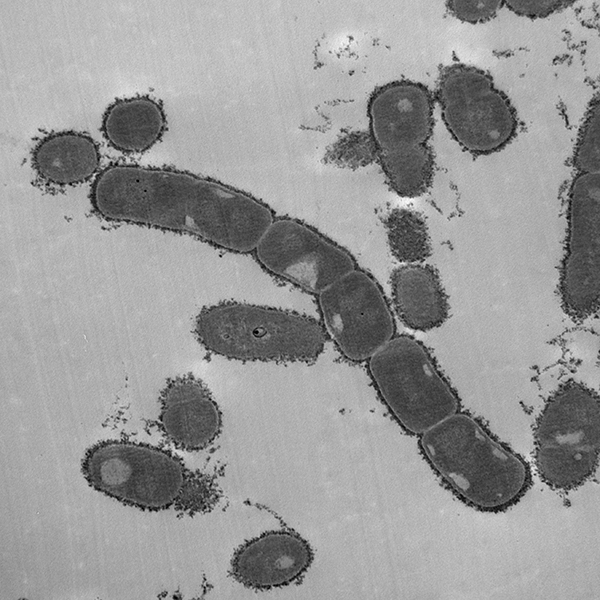
K. kingae expresses both a polysaccharide capsule as well as an exopolysaccharide, surface factors that together confer resistance to complement-mediated killing and neutrophil phagocytosis and are required for full virulence in an infant rat model of infection. Each isolate contains genes to produce one of four capsular types (a, b, c, or d). Previous work by the St. Geme Lab has shown that the type a and b capsules are associated with invasive disease and that the type a capsule inhibits opsonization, ROS production by neutrophils, and bacterial association with neutrophils. Additionally, the type a capsule has been shown to interfere with pilus-independent adhesion. We are currently exploring the impact of the other three capsular types on adherence to host cells, interaction with neutrophils, and virulence in animal models.
Recent work in the lab has shown that an additional surface polysaccharide known as an exopolysaccharide galactan increases bacterial resistance to opsonization, confers resistance to antimicrobial peptides, and reduces neutrophil phagocytosis. We are currently conducting studies to determine how the galactan is anchored to the cell surface and to elucidate the genetic basis and biochemical basis of exopolysaccharide biosynthesis. Furthermore, we are interested in both the capsule and the exopolysaccharide as potential vaccine antigens to generate a broadly protective K. kingae vaccine.
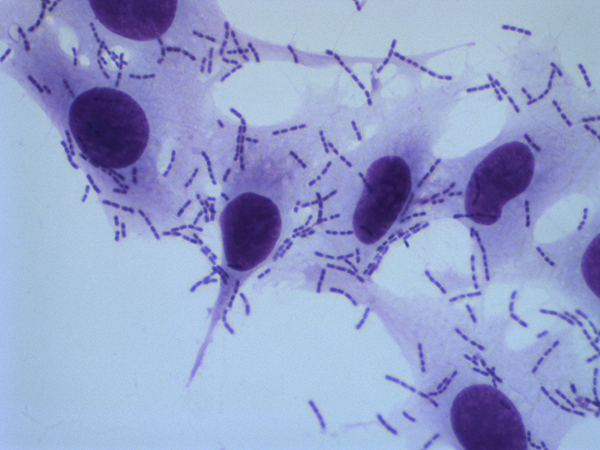
A critical first step in K. kingae pathogenicity is bacterial adherence to the host oropharynx and oropharyngeal colonization. Adherence is facilitated first by type IV pili and then by Knh, a member of the trimeric autotransporter family. The bacteria adhere to host cells using two pilus-associated adhesins, PilC1 and PilC2. Once the pili bind to host cells, the retractive force displaces the capsule, exposing the Knh protein. Together, pili and Knh allow K. kingae to interact intimately with the host. Current projects in the lab are investigating the impact of PilC1 and PilC2 on determining bacterial tissue tropism. Ultimately, we hope to use what we learn from these studies to develop novel strategies to treat or prevent K. kingae disease.
All known isolates of K. kingae contain at least one copy of the gene encoding RtxA, a broadly active toxin. This toxin is highly active against red blood cells, epithelial cells, synovial cells, and macrophages in vitro and is required for virulence in vivo in an infant rat model of infection. In recent work, the St. Geme Lab has identified a large clade of isolates that have undergone a gene duplication event, resulting in two copies of the toxin gene in the genome. These two-copy isolates are strongly associated with invasive disease. The St. Geme Lab is working to understand the impact of this duplication on toxin production, activity, and bacterial breach of epithelial barriers.
Nontypeable Haemophilus influenzae
Nontypeable H. influenzae (NTHi) is a common cause of otitis media and sinusitis in children and is also an important etiology of exacerbations of underlying lung disease, such as cystic fibrosis, bronchiectasis, and chronic obstructive pulmonary disease. Isolates of NTHi express a variety of adhesive proteins that interact with the host respiratory epithelium and facilitate the process of colonization. Examples under study in the St. Geme Lab include HMW1, HMW2, Hia, and Hap, all of which are transported to the bacterial surface via the type V secretion system as either members of a two-partner secretion system or autotransporters.
HMW1 and HMW2 are highly homologous glycoproteins that are prototypic members of a two-partner secretion system and are translocated across the outer membrane by cognate outer membrane proteins called HMW1B and HMW2B, respectively. The HMW1 and HMW2 adhesins form short helical fibers on the bacterial surface and facilitate high levels of adherence to cultured human respiratory epithelial cells.
HMW1 and HMW2 are glycosylated by a glycosyltransferase that is encoded by the hmw1C and hmw2C genes. Glycosylation is essential for proper display of the mature adhesins on the bacterial surface and for adhesive function. In ongoing research, the St. Geme Lab is investigating the recognition between HMW1 and HMW1B and between HMW2 and HMW2B, the purpose of glycosylation of HMW1 and HMW2, and the conservation of HMW1C-like glycosyltransferases in other gram-negative bacteria. We are also examining the immunologic properties and the vaccine potential of HMW1 and HMW2.
The Hia adhesin is a trimeric autotransporter and is present in approximately 25% of nontypable H. influenzae strains, including almost all H. influenzae strains that lack HMW1 and HMW2 adhesins. Like other trimeric autotransporters, Hia has an N-terminal signal peptide, an internal passenger domain, and a C-terminal translocator domain (also designated a ß-domain) and folds into a trimeric structure with three identical faces. The Hia passenger domain harbors two homologous binding domains referred to as HiaBD1 and HiaBD2 and mediates high affinity adherence to human respiratory epithelial cells.
The presence of three identical faces provides potential for three identical binding pockets and a multivalent interaction with the host cell surface, resulting in increased avidity and more stable interaction compared with a monovalent interaction. This increase in avidity may be especially important in allowing organisms to overcome mechanical forces in the host, including the mucociliary escalator, coughing, and sneezing. Beyond facilitating multivalent binding, a trimeric architecture may also confer stability to proteases and detergents. In ongoing research, the St. Geme Lab is investigating the Hia host cell receptor and are examining the relationship between Hia-mediated adherence and epithelial cell cytokine production.
Hap is a conventional autotransporter protein and has an N-terminal signal peptide, an internal passenger domain, and a C-terminal translocator domain in a monomeric architecture. This protein was first identified based on its capacity to promote bacterial adherence and entry in assays with cultured human epithelial cells. In addition, Hap mediates bacterial adherence to extracellular matrix proteins and bacterial aggregation and microcolony formation. The C-terminal 511 amino acids of the Hap passenger domain are responsible for interactions with fibronectin, laminin, and collagen IV, and the C-terminal 311 amino acids of the Hap passenger domain are responsible for interactions with epithelial cells and for mediating Hap-Hap interaction, triggering bacterial aggregation and microcolony formation.
Hap also contains an N-terminal serine protease domain, with a canonical chymotrypsin family catalytic triad corresponding to His98, Asp140, and Ser243. The serine protease domain mediates autoproteolysis and release of the Hap passenger domain from the bacterial surface, modulating bacterial adherence and aggregation. Autoproteolytic activity is inhibited by secretory leukocyte inhibitor (SLPI), a serine protease inhibitor that is present in varying amounts in the upper and lower respiratory tract and that results in enhanced Hap adhesive activity.
In ongoing work, the St. Geme Lab is examining the relationship between Hap-mediated microcolony formation and biofilm formation, the mechanism by which lipopolysaccharide biosynthesis affects Hap stability in the outer membrane, and the influence of Hap on HMW1- and HMW2-mediated adherence.