HOW CAN WE HELP YOU? Call 1-800-TRY-CHOP
Clinical Vector Core Services
Ready to Get Started?
Contact our Clinical Vector Core experts for a consultation and learn how we can help bring your cell and gene therapy projects from the laboratory to the patient's bedside, or complete the Vector Request Form for highly customized viral vector manufacturing services compliant with current Good Manufacturing Practices.
The Clinical Vector Core (CVC) offers Good Laboratory Practices (GLP)-grade adeno-associated virus (AAV) and lentiviral (LV) vectors in addition to current Good Manufacturing Practices (cGMP)-grade viral vectors in support of early phase clinical trials. GLP-grade products are manufactured using a GMP-comparable process to support pre-clinical pharmacology and toxicology studies and early-phase clinical trials.
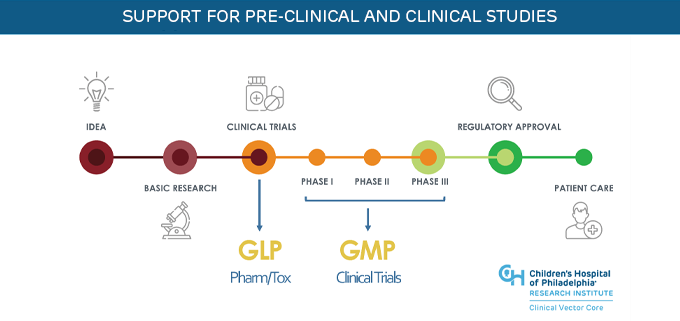
The CVC offers the following services and quality control measures, refined over nearly two decades of vector manufacturing experience.
Services
- Manufacturing for LV vectors and AAV vectors
- AAV vectors include a variety of standard AAV serotypes, including AAV 1, 2, 5, 6, 8, and 9, as well as customer-defined custom serotypes (novel or modified serotypes may require a pilot run prior to scale-up)
- Long-term stability testing services consistent with the duration of the Phase 1 and 2 study
- Device compatibility and short-term stability studies
- Research-grade products for proof-of-principle and bridging studies
- Clinical products are manufactured in compliance with FDA cGMP regulations applicable to Phase 1 and 2 clinical trials
- Manufacturing of GMP-grade and non-GMP grade AAV excipient
- Regulatory and Chemistry, Manufacturing and Control (CMC) support for Investigational New Drug (IND) and Investigational Medicinal Product Dossier (IMPD) applications
- Letter of cross reference to our Drug Master Files
- Other (please piecyks [at] chop.edu (contact us) for a full list of release assays)

Quality Control
- Bioburden/Sterility
- Bacteriostasis/Fungistasis
- Mycoplasma
- Endotoxin
- Replication Competent AAV
- Replication Competent LV
- Adventitious agents
- Other
